The Dissolution of Calcium Hydroxide Is Exothermic
Pools of calcium hydroxide either passive or agitated provide one of the simplest concepts in which CO 2 from air can be captured with calcium carbonate precipitating and accumulating. The initial pH did not significantly affect the phenol removal efficiency in 120 min although k decreased with an increase in pH Fig.

Solved 19 The Dissolution Of Calcium Hydroxide Is Chegg Com
2a Table S5The phenol removal efficiency was 980 and 9445 at an initial pH of 3 and 11.
. Calcium carbonate must then be separated and dried and must undergo a process known as calcination at temperatures above 700 C to form calcium oxide with the release of. Hot spots for highly exothermic reactions making temperature easier to control 4 favoring lower-order reactions in parallel reaction schemes 5 economical operation. This includes sodium oxide potassium oxide calcium oxide magnesium oxide and aluminium oxideThe oxides must be molten before immersing graphite electrodes in them.
2Al 2 O 3 4Al 3O 2 Hydrolysis and dissolution. The effect of the initial pH of solution on phenol removal was investigated by adjusting the initial pH from 3 to 11 with 01 M HCl or NaOH. Since metals that are reactive form oxides that are stable some metal oxides must be electrolyzed to be reduced.

Solved 13 The Dissolution Of Calcium Hydroxide Is Chegg Com

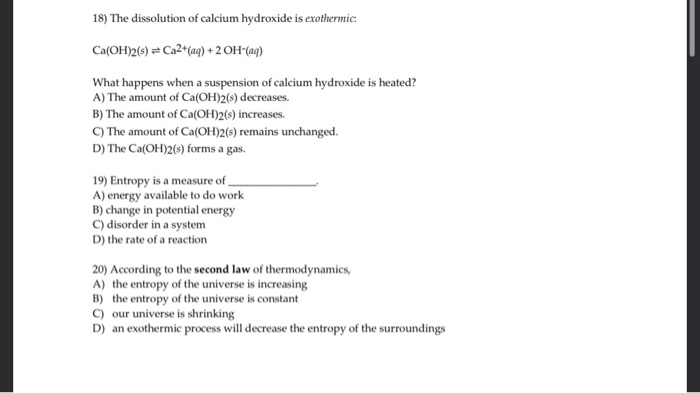
Solved 18 The Dissolution Of Calcium Hydroxide Is Chegg Com
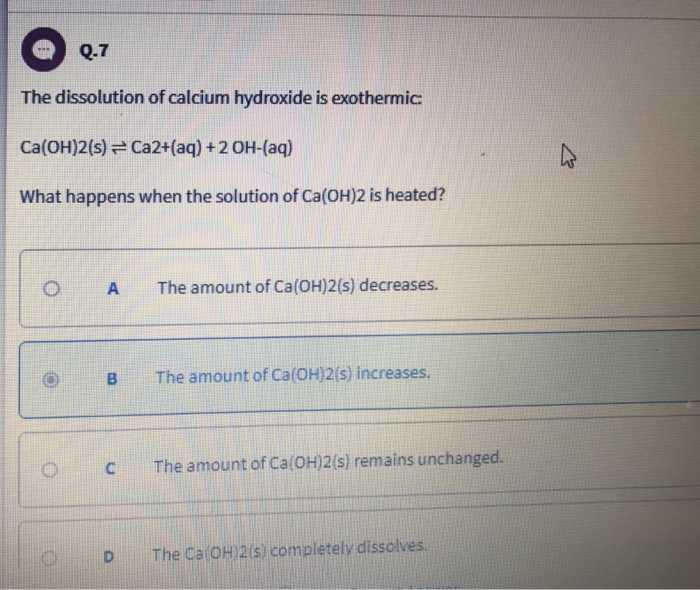
Solved Q 7 The Dissolution Of Calcium Hydroxide Is Chegg Com
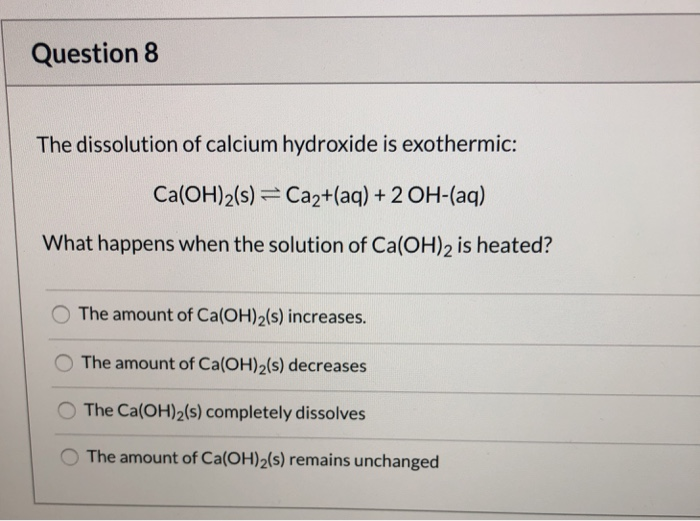
Solved Question 8 The Dissolution Of Calcium Hydroxide Is Chegg Com
Comments
Post a Comment